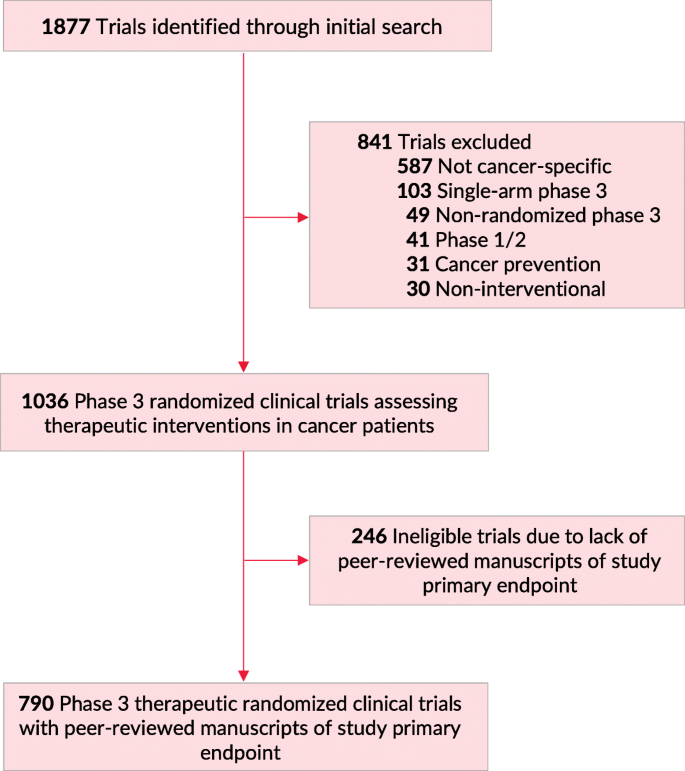
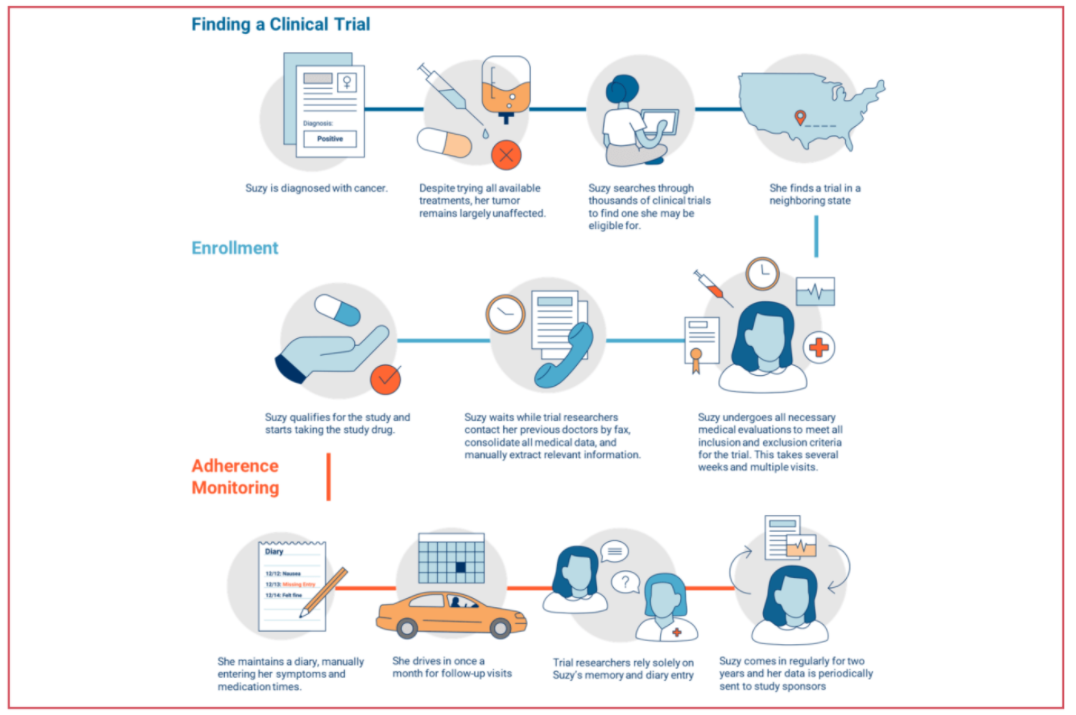
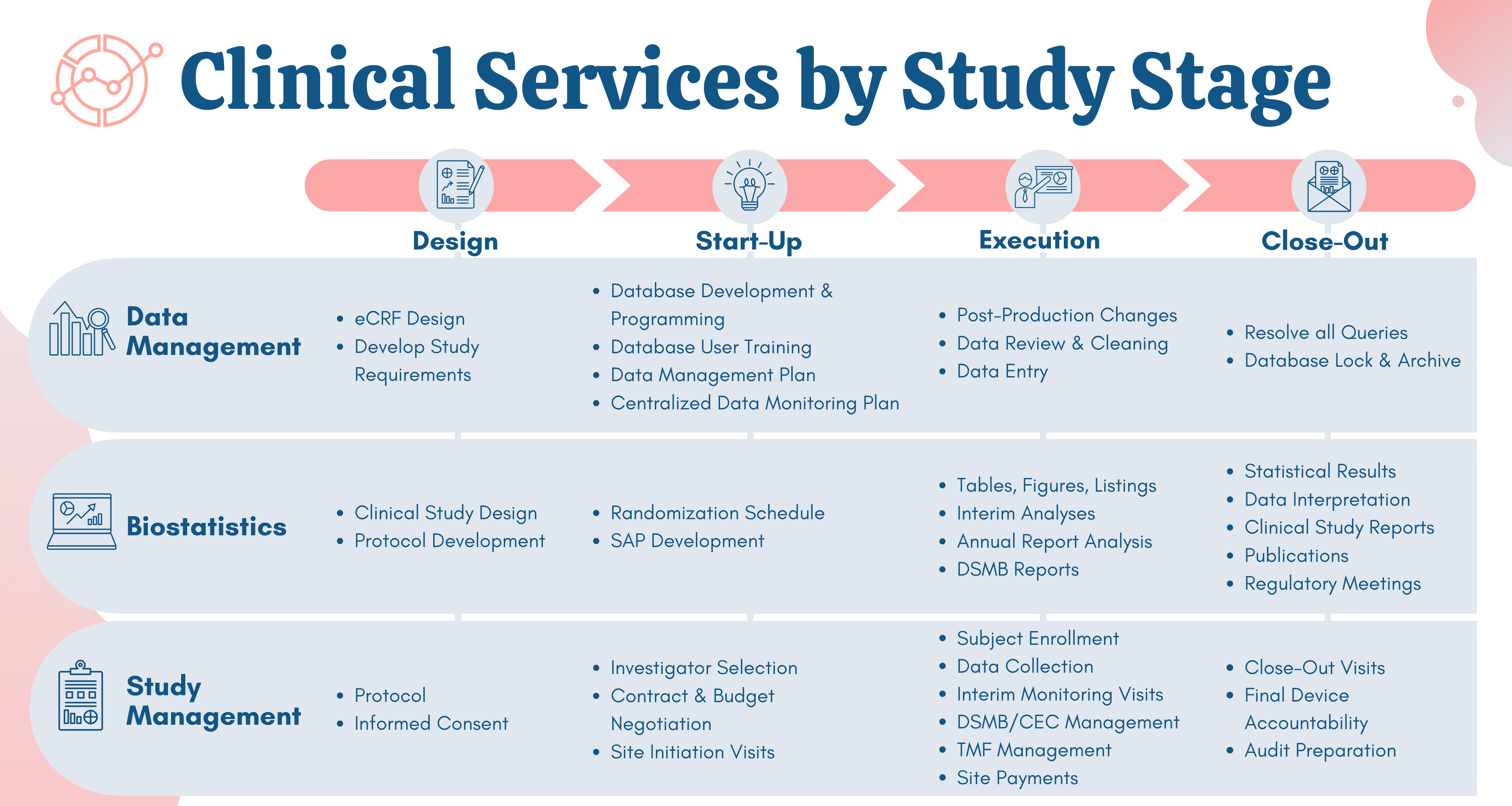
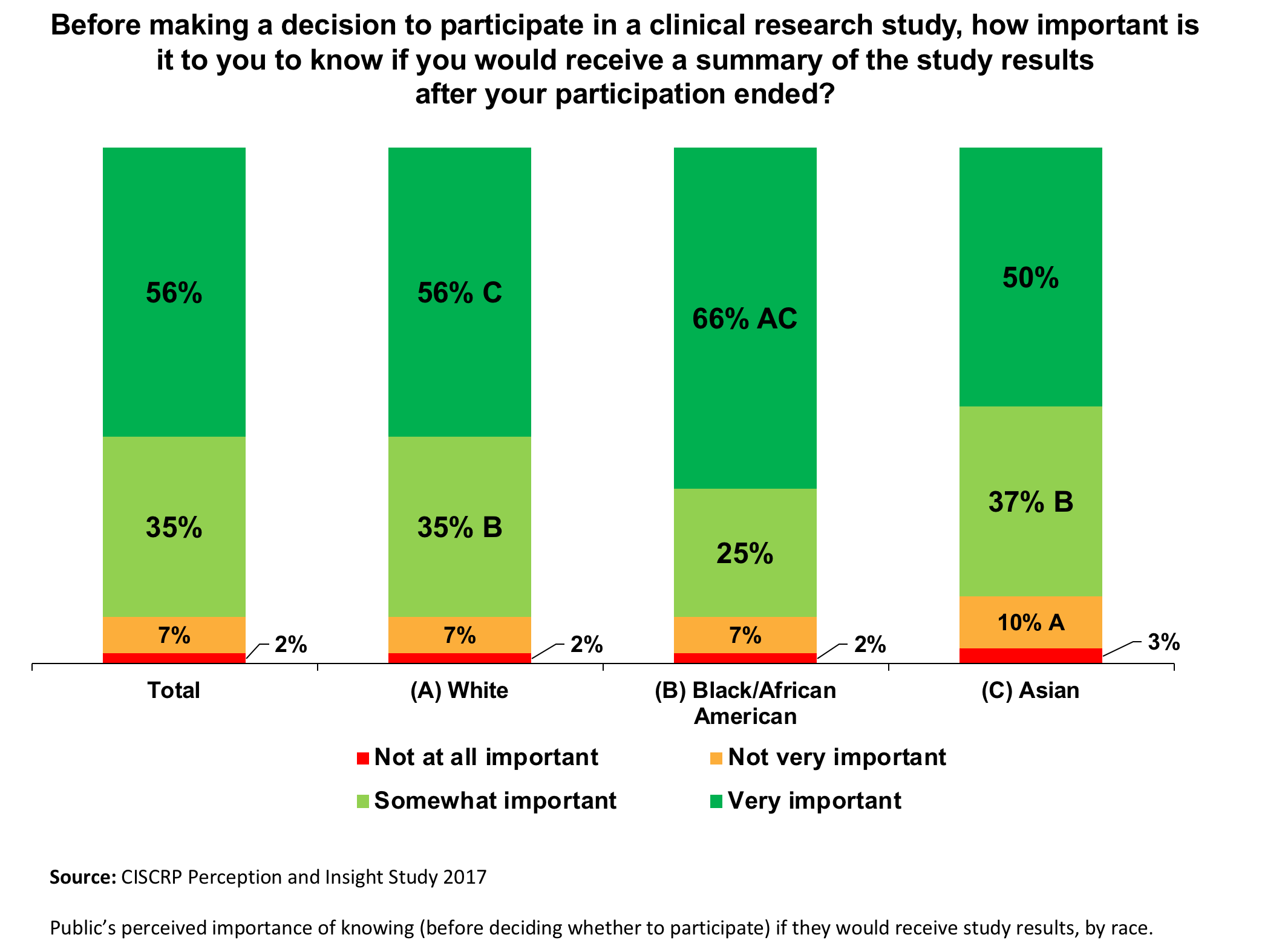
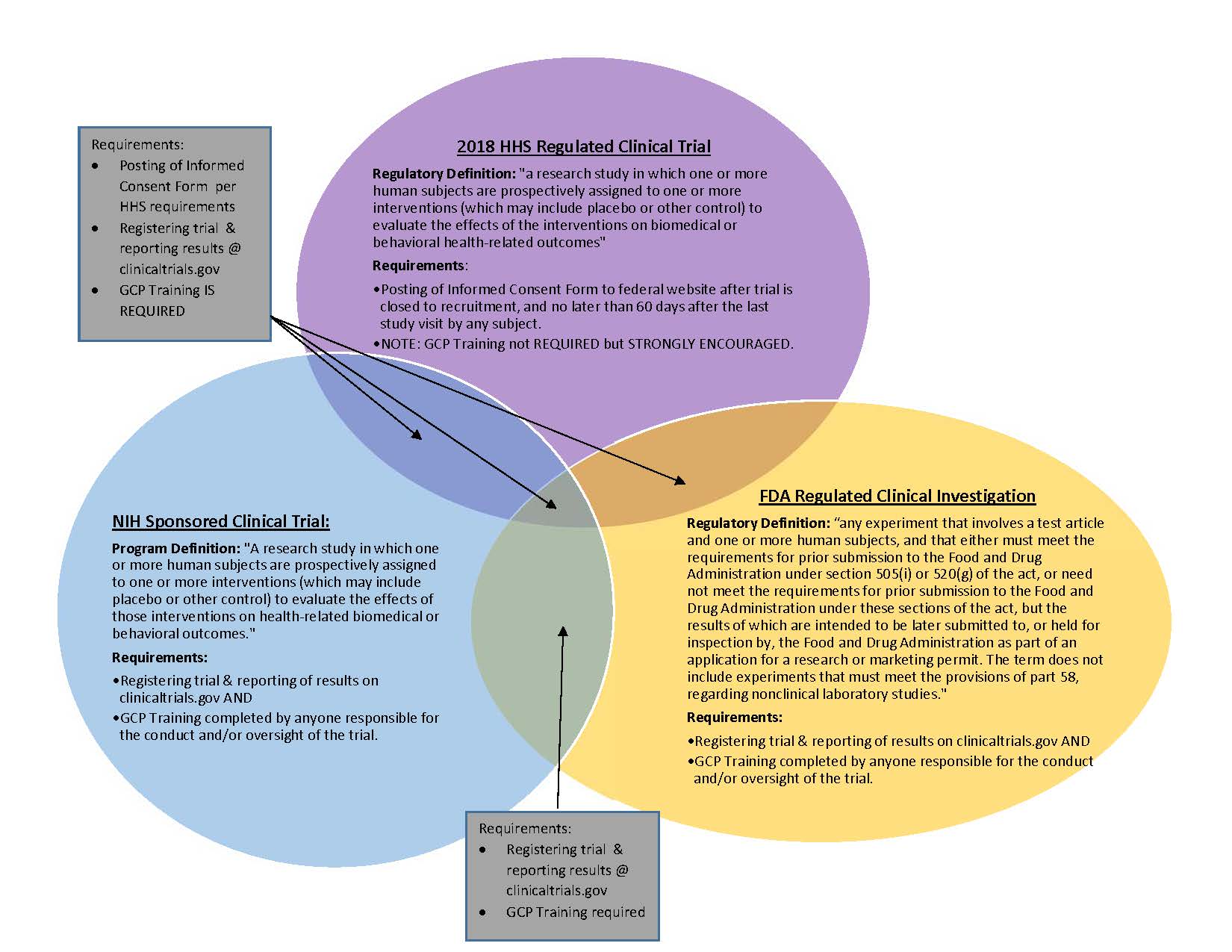
Clinical trials site recruitment optimisation: Guidance from Clinical Trials: Impact and Quality - Christine Zahren, Sonia Harvey, Leanne Weekes, Charlotte Bradshaw, Radhika Butala, John Andrews, Sally O'Callaghan, 2021

PRESERVE: Randomized Trial of Intensive Versus Standard Blood Pressure Control in Small Vessel Disease | Stroke
Evidence for the Selective Reporting of Analyses and Discrepancies in Clinical Trials: A Systematic Review of Cohort Studies of Clinical Trials | PLOS Medicine

Design characteristics, risk of bias, and reporting of randomised controlled trials supporting approvals of cancer drugs by European Medicines Agency, 2014-16: cross sectional analysis | The BMJ